- Posts: 224
- Thank you received: 0
30" of snow lost in 21hrs @ Snoqualmie?
- Telemon
-
- User
-

Please Log in or Create an account to join the conversation.
- Eric_N
-

- User
-

- Posts: 42
- Thank you received: 0
"I am therefore compelled to beleive that the snowey mountains yeald their warters slowly, being partially effected every day by the influence of the sun only, and never suddonly melted down by haisty showers of rain."
-Meriwether Lewis-
Friday June 14th 1805, Missouri River, near present day Great Falls, Montana {quote and spelling}
Sunny and crowded like buffalo at Alpental this morning.
Please Log in or Create an account to join the conversation.
- skierguitarist
-

- User
-

- Posts: 64
- Thank you received: 0
I also think that the 30" decrease in snowdepth is almost entirely due to settlement (compaction) and not melting.
Years ago I mentioned this notion to some friends--most were non-believers.... Thanks for the wonderful academic explanation Amar!!! Fantastic!!!!!!!
(or is it fear, for skiers?) that when rain falls on a snowpack, it must be melting the snow. The fact is that rain, especially when it falls within a few degrees C of the freezing point, is almost entirely incapable of melting snow.
What, you might say? No way... But the simple reason (if you remember from science class) is the huge latent heat of fusion , which is the amount of heat (= energy) needed to melt a given quantity of snow or ice into liquid. For water, this energy for melting is roughly the same amount of energy as required to warm the same quantity of water by 80 degC, nearly to the boiling point.
So to just barely melt a kilogram of snow at the freezing point (0 degC), we'd have to put it in a pot and pour 1 kilogram (=1 liter) of 80 degC water onto it, and that water would be cooled all the way down to 0degC in the course of melting the snow. If we decided to try using 5 degC (= 41 degF) water, we would need 16 liters of it (80/5) in order to melt the same kilogram of snow. Using 1 degC (34 degF) water, we'd now need an astonishing 80 liters of it to melt the snow.
Now back to snowpack and rainfall: it's easier to think in terms of inches of snowdepth or precip instead of kilograms, so let's switch to that. How much rain fell at Snoqualmie Pass over 2 days, about 10" at close to 41 degF, right? That amount of rain would only be capable of melting 10/16 = 0.6" of snow-water equivalent (SWE). The 74" of snowpack prior to the rain probably had a density of roughly 25%, so it contained about 18" of SWE, and the rain could only have melted 0.6" of that, a fairly insignificant amount.
What about melting due to the actual warm air temperature? Again, air has a very hard time melting snow, even more so than rain because the heat capacity of air is about 4.2 times less than that of water. Therefore 4.2 times as much mass of air is needed to perform the same melting as a given mass of water. So melting a kilogram of snow using air at 5 degC (41 degF) would require 66 kilograms of it (330/5), which is a volume of about 55 cubic meters (almost 2000 cu ft), or the volume of a 12 x 20 ft room with 8 ft ceilings. And all that air would be cooled right to 0 degC in the process of melting 1 measly kilogram of snow. It's easy to see why strong winds are key to melting snow rapidly, because you've got to keep a continual fresh supply of 40 degF air coming through if you're going to melt any significant snow. With calm winds, the air right above the snow is quickly cooled to 0 degC, and it just sits there, incapable of melting any snow at all. The warmer air farther above is insulated from the snow by the denser now-cooled air, and without wind (or solar heating) the cold dense air can't move out of the way.
So two days of 40 degF temperatures probably melted even less of the snow than did 10" of rain at that temperature, but trying to calculate this is not easy. Let's just say it's lost a total of about 1" of SWE out of 18". With the current 44" depth and now 17" of SWE, that gives a density of about 40%, which makes perfect sense. That's the same as the typical springtime density of a well-consolidated maritime snowpack. Instead of having an unusually light-and-fluffy snowpack as we did before this storm, it's now settled to more typical Pacific Northwest density. But very little of it, only a few %, has actually been lost due to melting.
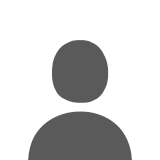
But how can we verify that any of these calculations are even close to correct? We need some way of knowing the actual SWE in the snowpack. Thankfully, even though NWAC doesn't use them, the NRCS SNOTEL network has a SWE sensor at every one of its sites (it's a rubber pillow filled with antifreeze, set flush to ground level, with a pressure sensor to measure the weight of the snowpack sitting on it). We just need to find a nearby SNOTEL site at a similar elevation, about 3000 ft: let's pick Cougar Mountain SNOTEL at 3200 ft (NOT the same as the Cougar Mountain near Issaquah), which has decent data (no big gaps or missing values like most other nearby sites) and this map shows how close it is to Snoqualmie Pass. I grabbed the last 7 days of hourly data and plotted it:
The green line shows that about 10" of precip have fallen the last 2 days, with temps (cyan line) around 40-43 degF. The blue line shows the rapidly decreasing snowdepth over the past 2 days, while the black line is the water content of the snow (SWE). Even though the depth has decreased from 50" to 37", the SWE has barely decreased at all, dropping only from about 17" down to 16". Meanwhile, the density (violet line) at this site has increased from 31% to 44% over the same time. Which illustrates numbers comparable to those calculated above, verifying that the basic point of this post is true: The snowpack at 3000 ft in the Central Cascades has settled during this Pineapple Express, but not melted in any significant way. Despite all the rain, only a few percent of the pre-existing snowpack has melted, well under 10% loss even at this fairly low elevation.
(Note that this SNOTEL site is west of the crest and isolated from any very cold easterly pass flow like Snoqualmie Pass gets, so it makes sense that the Snoqualmie Pass site would have had a much lighter-density snowpack prior to the rain, and thus settled more during the rain. Maybe John, i.e. Stimbuck, has the actually density numbers from his pit?)
Please Log in or Create an account to join the conversation.
- andyrew
-

- User
-

- Posts: 116
- Thank you received: 0
It has another good explanation of the dangers snow-sucking fog pose to the snowpack compared to sun or rain.
Please Log in or Create an account to join the conversation.
- Stugie
-

- User
-

- Posts: 291
- Thank you received: 0
Please Log in or Create an account to join the conversation.
- Salal
-

- User
-

- Posts: 53
- Thank you received: 0
Please Log in or Create an account to join the conversation.